sodium sulfite
Specifications
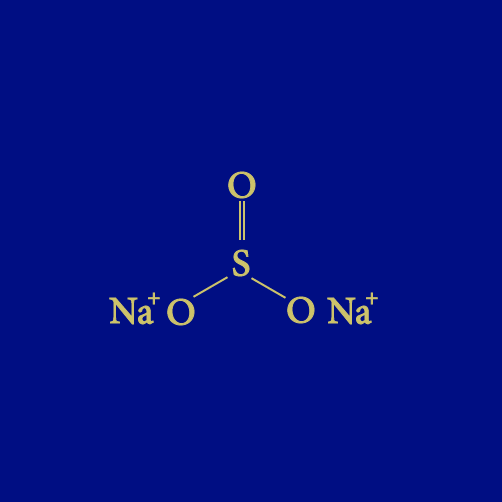
Chemical formula: Na2SO3
Molecular weight: 126
Appearance: white to yellowish powder
20% solution: colorless and transparent or yellowish
Packaging: 25 kg, double-layer polyethylene bags
Chemical analysis
| Technical grade | Food/Photographic grade* | |
|---|---|---|
| Purity(Max.) | 90% | 97% |
| Alkalinity (as Na2CO3) (Max.) | 0.3% | 0.015% |
| Iron (Max.) | 0.005% | 0.002% |
| Amount of Ca and Mg and other substances insoluble in ammonia (Max.) | ---- | 0.5% |
| Sodium thiosulfate (Max.) | ---- | 0.015% |
| Heavy metals (as Pb) (Max.) | ---- | 0.002% |
| Ammonia silver nitrate test | ---- | Passed |
| 200 g/liter solution | ---- | It does not settle and is not cloudy |
* This product complies with ISO 418 standard (photographic grade sodium sulfite).
Applications
It is used in various industries for the following purposes:
- In the water purification industry to remove excess oxygen and chlorine from water and prevent corrosion, especially in steam boilers and brine purification systems (sea water evaporators).
- In oil extraction operations in order to remove oxygen from water to prevent corrosion in oil well equipment and in drilling mud.
- In the leather industry, as an auxiliary chemical in the leather production process (and sulfation process).
- In the mining industry, as an auxiliary material in the flotation of some mineral materials.
- In the chemical industry, in order to produce sodium thiosulfite, sulfonation, sulfomethylation, and reduction.
- In the food industry, as a food preservative.
- In the photography industry, in the formulation of proven and emerging drugs.
- In the paper industry, in some paper production processes such as semi-chemical and chemical-thermal-mechanical processes.
- In the textile industry, in bleaching wool, polyamid fibers and removing excess chlorine after chlorination processes.
- In the chloralkali industry, in order to remove excess chlorine.
- In the detergent industry, in order to produce sulfosuccinates (the basic ingredient of baby shampoo).
- In the rubber industry, in order to stabilize the latex.